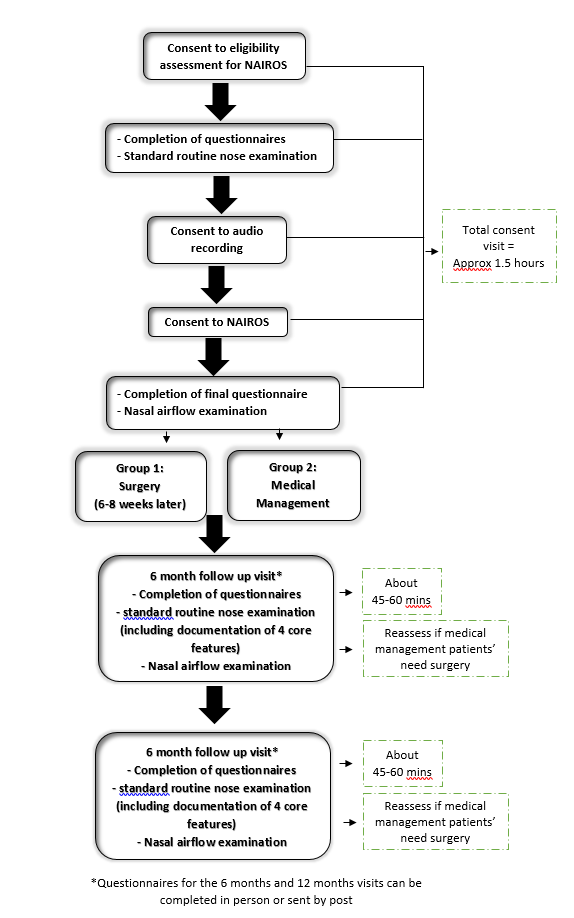
How will the study be carried out?
Visit 1:
- Participants are examined by their consultant to assess their eligibility. A thin endoscope (telescope) is used to look at the nasal passages, and participants complete two short questionnaires on their nasal symptoms.
- Once participants are confirmed to be eligible, further assessments will be undertaken. The participant is asked to breathe through two nosepieces and the airflow from each side of the nose is measured (Rhinospirometry).
- Participants are also asked to complete further short questionnaires about breathing and quality of life.
- Participants are randomised to Medical Management arm and are prescribed Mometasone nasal steroid spray and Sterimar saline spray for 6 months
OR
- Participants are randomised to surgery, and then undergo Septoplasty surgery, with or without unilateral turbinectomy (reduction of swellings on nasal side walls), within 2 months of the first visit. More information on septoplasty can be found here.
Visit 2:
- After 6 months, participants return to the clinic and are examined as above again.
- Participants are asked about any medical issues they have had in the past 6 months, and how they feel now. Participants are also asked complete short questionnaires about the financial impact of illness and treatment.
Visit 3:
- After 12 months, participants undergo a final clinical examination and are asked once more to complete questionnaires about nasal symptoms, quality of life and the financial impact of illness and treatment. Participants are again asked about any medical issues they have had in the past 6 months, and how they feel now.
By repeating the same tests at each visit, we can compare participants' condition before and after treatment for both surgery and medical management, to see if one has better outcomes for patients.
Flowchart of Study Process